 |
| Physics Today: Optical detection of radiocarbon dioxide (partial view) |
Topics: Modern Physics, Radiation, Spectrograph
A compact spectroscopic system can measure radioactive carbon dioxide concentrations as low as five parts per quadrillion.
Carbon’s only naturally occurring radioactive isotope, 14C, is exceedingly rare. Produced when neutrons from cosmic rays interact with nitrogen, the radioisotope makes up just one part per trillion of the carbon in Earth’s atmosphere. Yet because of its continual production, its diffusion through the planet’s carbon cycle, and its long half-life of 5700 years, 14C is routinely used to date organic matter as old as 50 000 years. Archaeologists, forensic scientists, and environmental researchers, among others, essentially measure the concentration of radiocarbon in a sample to determine its age.
Since the late 1970s, accelerator mass spectrometry (AMS) has served as the benchmark method for the job. In that approach, samples are burned, chemically converted to graphite, and bombarded with cesium ions. The negative carbon ions ejected from the solid samples are then accelerated to a few percent of the speed of light and their mass-to-charge ratios deduced from their trajectories through electric and magnetic fields. Fortuitously, the most common isotope of nitrogen in the atmosphere, 14N, forms no stable negative ion; and its absence eliminates its otherwise large interference with the 14C signal. Likewise, 12CH2 and 13CH molecules are broken apart during a later, electron-stripping stage and don’t survive to interfere with the signal.
Both effects help free 14C signals from background noise. But although the technique is powerful—and applicable to other trace elements—the spectrometers can cost millions of dollars and often require a dedicated facility to maintain their electrodes at hundreds of thousands to millions of volts in a vacuum.
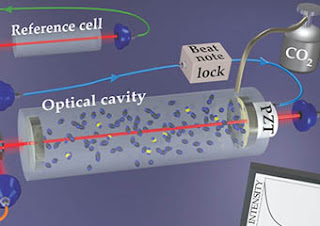
A technically simpler approach also begins with burning a sample, but only to transform its carbon atoms into carbon dioxide molecules. With their strong vibrational absorptions in the mid-IR, the many isotopic combinations of CO2 can be distinguished optically. The challenge is to measure the intensities of their spectral lines to determine the concentration ratios. The task is not easy if the goal is to count trace isotopes in a sea of abundant ones. The CO2 molecule has hundreds of vibrational and rotational lines, many of them closely spaced in frequency. And even the most stable lasers suffer from intensity fluctuations.
Physics Today: Smaller, faster, cheaper detection of radiocarbon, R Mark Wilson
Comments