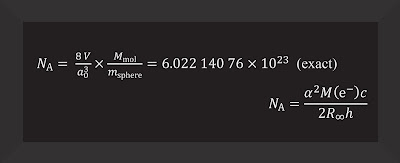 |
| Credit: Design: N. Hanacek/NIST |
Topics: Chemistry, Education, NIST, Periodic Table, STEM
In suburban Maryland, on the third floor of the Advanced Chemical Sciences Laboratory at the National Institute of Standards and Technology (NIST), Bob Vocke and Savelas Rabb are explaining how they are helping to redefine the mole, that mammoth concept we learned in high-school science class.
Mostly, this means using abstract symbols and numbers, dancing along in a long equation that Vocke obviously thinks is beautiful. You can tell because he enthusiastically writes each detail out on the white board for some lab visitors. Every so often, Rabb provides suggestions or tells a joke and sort of eggs Vocke on. It is clear they are having a good time and could do this kind of annotation and explanation all day.
After the mole equation, they add more:
 |
| Credit: Design: N. Hanacek/NIST |
To fully grasp the new definition of the mole, you must embrace these long equations, which show how that measurement connects to fundamental constants of the universe—such as the speed of light in vacuum and the amount of charge in an electron. In turn, these fundamental constants will completely redefine the modern metric system, known as the International System of Units (SI). The mole is one of the seven base units of the SI.
For Vocke and Rabb, the equation work brings joy. The process is entertaining, and a little fun.
For most others, it seems impossibly cryptic.
On the surface, the mole’s basic definition will remain the same. It’s a measure of stuff—how many molecules or atoms you have of a particular substance such as water, or gold or a protein.
But the current definition is more complicated than it needs to be. In the present metric system, a mole is the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of the most common form of carbon, known as carbon-12. A further complication: the mole relies on another definition, the definition of the kilogram, which is currently specified by the mass of a platinum-iridium cylinder locked up in a special vault outside Paris, France.
*****
Mole Day is an unofficial holiday celebrated among chemists, chemistry students and chemistry enthusiasts on October 23, between 6:02 a.m. and 6:02 p.m.,[1][2] making the date 6:02 10/23 in the American style of writing dates. The time and date are derived from Avogadro's number, which is approximately 6.02 × 1023, defining the number of particles (atoms or molecules) in one mole (mol) of substance, one of the seven base SI units.
Mole Day originated in an article in The Science Teacher in the early 1980s. Inspired by this article, Maurice Oehler, now a retired high school chemistry teacher from Prairie du Chien, Wisconsin, founded the National Mole Day Foundation (NMDF) on May 15, 1991. Wikipedia
Redefining the Mole, NIST
Spectrometers, Silicon Spheres and Statecraft Modernize Chemistry’s Mammoth Measurement Unit
Comments