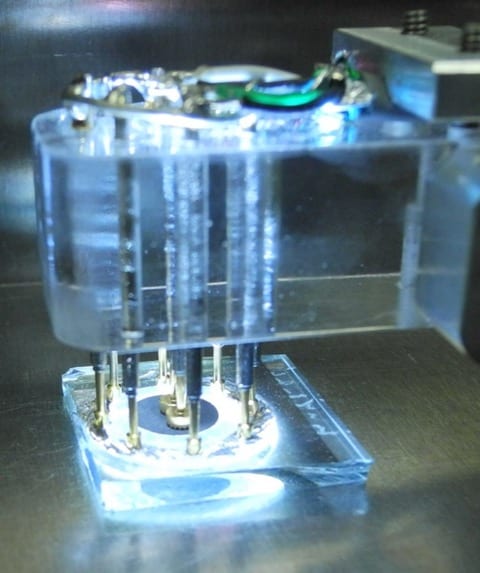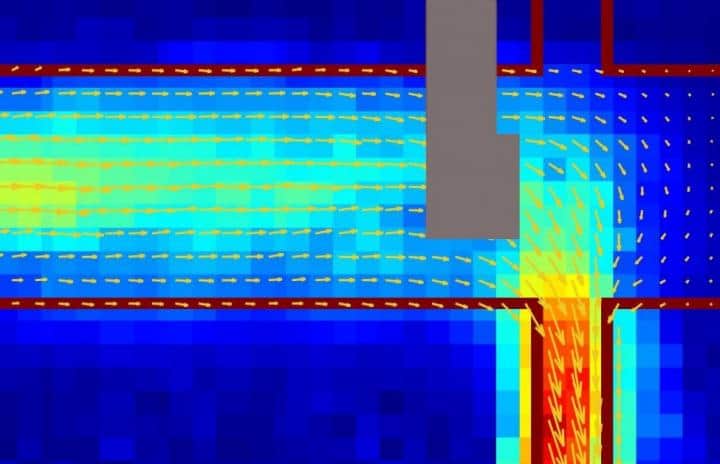
A picture of an electrical current in graphene (marked by the red outline) showing a fluid-like flow imaged using a diamond-based quantum sensor. The grey portion is where the metal electrical contacts prevented collection of data. Courtesy: Walsworth and Yacoby research groups, Harvard and University of Maryland
Topics: Materials Science, Nanotechnology, Quantum Mechanics, Semiconductor Technology
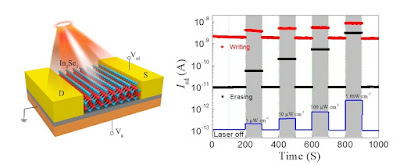
A team led by researchers from Harvard University and the University of Maryland in the US has used defects in diamond to map the magnetic field generated by electrical currents in graphene. Their experiments reveal that currents in this atomically-thin form of carbon flow like a viscous fluid – a result that could provide fresh insights into the collective behavior of electrons in strongly-interacting quantum systems.</em>
Graphene has many exceptional electrical properties. Among them is the fact that, at the point where its conduction and valence bands just touch each other (the Dirac point), it can support currents composed of electrons and an equal number of positively-charged holes, rather than electrons alone. In the present work, Ronald Walsworth, Amir Yacoby and colleagues set out to establish whether these electron-hole plasmas (or Dirac fluids, as they are also known) flow smoothly, like electrons traveling through a metallic wire, or unevenly like water running through a pipe.
Diamond defects reveal viscous currents in graphene, Isabelle Dumé, Physics World