working on a Magical Girl concept
Featured Posts (3520)
It's Fight Friday! In honor of the new Wolverine trailer we are pitting him against a wildcard. Who do you think would win?
 |
| Image Source: Pinterest - The Twilight Zone |
Topics: Commentary, Politics, Science Fiction
I miss television shows like this. Television - before the special effects and hyped violence - was an introspective experience, plots took time to develop because people actually read more for enjoyment and expected a similar pacing. Things like horror and thrills weren't created for voyeuristic consumption: most of the greatest thrills were completely fabricated in your mind. I missed this quite literally and existentially by five months (and perhaps a few years to develop language and comprehension), gestating in my mother's womb. I would see it much later in syndication.
"Little Girl Lost": the video has a weird blue background on YouTube, so I just give the link. A fourth dimension seemed exotic then, well after Einstein's Special Theory of Relativity established spacetime as four dimensions, the fourth being time. The notion of other dimensions beyond even these was at least thought of philosophically well before anyone thought of String Theory and multiple dimensions as is now theorized. It was an age where the United States was in a pitched battle called the Space Race, an extension of the Cold War with the USSR. It was an age of dichotomy - fear and suspicion; wonder and adventure: The Invaders, Lost in Space, Star Trek, The Time Tunnel were actual prime time popular science fiction shows along with The Twilight Zone.
Here we are: in the 21st Century, the notion of reasoned, scientific inquiry is suspect to manipulation, politicization, "conspiracy" allegations and charges of fraud. Whole screeds are posted to the Internet on the "folly" of climate science in particular or science in general without the slightest notion of the powerful irony of their actions.
In the American Physical Society article by James Kakalios (that inspired this post):
The March 1962 episode “Little Girl Lost” of the television anthology program The Twilight Zone added some speculative inter-dimensional physics to a suspenseful science fiction tale.[1] In this story a small child rolls out of her bed in the middle of the night and disappears. Her parents become frantic when they can hear her calls for help, but cannot see or touch her. Fortunately they know what to do in just such an emergency — they call for their neighbor Bill, who is a physicist. He determines that the girl has accidentally fallen through a portal into another dimension. With his aid, and the help of the family dog, they manage to retrieve their daughter. Whether this portal was to one of the extra dimensions predicted by String Theory is open to interpretation, but the show clearly demonstrated the utility of a friendly neighborhood physicist.
Indeed, in the early 1960’s, the U.S. Government had similarly concluded that it was worthwhile to have physicists and other scientists on call. Following the Manhattan Project; the development of radar; and the proximity fuse in World War II the value of scientists and engineers to national security was accepted by the general public. In 1942 West Virginia Senator Harley Kilgore had proposed legislation calling for federal support of scientific research and in 1945 Vannevar Bush’s report Science, The Endless Frontier, [2] forcefully argued that it was in the nation’s best interest to develop and maintain strength in what we now would refer to as STEM fields. In 1950 Congress responded with the establishment of the National Science Foundation.
The situation today is very different. There is no longer broad agreement among the public of the value of scientific research.[3] Which is ironic, for this same public has enthusiastically embraced personal electronics and technology that is enabled, in part, through federally funded research. As expressed a few years ago by a Dean at M.I.T., never before in human history have so many become so wealthy solely through education. [4].
Metaphorically, we are nationally that little lost girl, or more gender-neutral: children. The world is as it has always been. There weren't transistor tubes before semiconductors; there weren't transistor radios before boom boxes, walk-men, CD players or cell phones with apps. The Internet wasn't a concept by ARPA/DARPA in 1964 before it became DARPANET soon after 1984 when I was a commissioned Communications Officer in the Air Force. There was no file transfer protocol (FTP); or hypertext markup language (HTML): we've always had Dreamweaver. We will always have fossil fuels as long as we have drills, liquid and fracking to force them from their depths. We will never as a planetary system - Earth - be subject to the Second Law of Thermodynamics as over time other systems (like our bodies) go from order to chaos.
The sanest reason for outreach: to find and reach humanity's children guiding them en masse socially to at least technological adolescence. So we, collectively will no longer "be lost." Dr. Martin Luther King was famous for a lot of things, but this quote was the most poignant and jarring, as the latter part of it is an obvious; glaring choice:
"Nothing in all the world is more dangerous than sincere ignorance and conscientious stupidity."
APS Physics: Why Do Outreach? James Kakalios
Topics: Applied Physics, Big Bang, Carl Sagan, Research
The clean room has an interesting history. In this recap on Space.com by Miriam Kramer (April 21, 2014, excerpt below):
The scientist who discovered the age of the Earth also helped end the use of lead in gasoline and other products in the United States.
Sunday night's episode (April 20) of "Cosmos: A Spacetime Odyssey" explored the life of Clair Patterson, a geochemist who pinpointed Earth's age for the first time and also uncovered a secret: Lead contamination is a major and potentially deadly problem. The newest episode of "Cosmos," called "The Clean Room," takes viewers on a tour of Patterson's work and the industry that fought him as he tried to learn more about lead and its harmful effects.
You can see more at the link. I have an "affection" for clean rooms (obviously) due to spending a considerable amount of time in them for things like your I-Phone, your I-Pad; your game platform, your GPS...etc.
| Moi... |
I guess I shouldn't be amazed that a facility first built to estimate the age of the Earth, then suddenly find out about lead poisoning in gasoline could also be used in clearly more imaginative ways. Clean rooms are used by NASA and ESA to assemble spacecraft prior to launch. It's almost poetic that they would have a usage on Earth to peer at the very epoch of the universe itself.
It takes a very, very clean room to build a detector sensitive enough to see the light from the beginning of the universe.
Work is underway at the U.S. Department of Energy's (DOE's) Argonne National Laboratory on a new "clean room." The new lab will be specially suited for building parts for ultra-sensitive detectors — such as those to carry out improved X-ray research, or for the South Pole Telescope to search for light from the early days of the universe.
"This will be a unique facility, and a wonderful investment for the future of the laboratory," said Supratik Guha, who heads the Center for Nanoscale Materials, a DOE Office of Science User Facility adjacent to where the new space will be located.
“We are a way for the cosmos to know itself.”
― Carl Sagan, Cosmos
Argonne National Laboratories:
Building a room clean enough to make sensors to find light from the birth of the universe
Louise Lerner
 |
| Image Source: ESA Space Science |
Topics: Astronomy, Astrophysics, ESA, Mars, Planetary Science
(Oct 16) A Mars lander left its mothership on Sunday after a seven-month journey from Earth and headed toward the red planet's surface to test technologies for Europe's planned first Mars rover, which will search for signs of past and present life.
The disc-shaped 577-kilogramme (1,272 lb) Schiaparelli lander separated from the spacecraft Trace Gas Orbiter (TGO) at 1442 GMT (10:42 a.m. EDT) as expected, starting a three-day descent to the surface.
Signals received from TGO, which is to orbit Mars and sniff out gases around the planet, did not at first contain data on the lander's onboard status, but the European Space Agency (ESA) later said the link with the craft had been restored.
#P4TC: ExoMars...
Reuters: European-led Mars lander starts descent to red planet
Reporting by Maria Sheahan; Editing by Dominic Evans, Greg Mahlich
Topics: Applied Physics, Condensed Matter Physics, Solid State Physics, Superconductors
Researchers at the U.S. Department of Energy's Ames Laboratory and partner institutions conducted a systematic investigation into the properties of the newest family of unconventional superconducting materials, iron-based compounds. The study may help the scientific community discover new superconducting materials with unique properties.
Researchers combined innovative crystal growth, highly sensitive magnetic measurements, and the controlled introduction of disorder through electron bombardment to create and study an entire range of compositions within a class of iron-based superconductors. They found that the key fundamental properties—transition temperature and magnetic field penetration depth—of these complex superconductors were dependent on composition and the degree of disorder in the material structure.
"This was a systematic approach to more fully understand the behavior of unconventional superconductors," said Ruslan Prozorov, Ames Laboratory faculty scientist and professor in the Department of Physics and Astronomy at Iowa State University. "We found that some proposed models of unconventional superconductivity in these iron-based compounds were compatible with our results, and this study further limited the possible theoretical mechanisms of superconductivity."Researchers combined innovative crystal growth, highly sensitive magnetic measurements, and the controlled introduction of disorder through electron bombardment to create and study an entire range of compositions within a class of iron-based superconductors. They found that the key fundamental properties—transition temperature and magnetic field penetration depth—of these complex superconductors were dependent on composition and the degree of disorder in the material structure.
Phys.org:
Scientists gain insight on mechanism of unconventional superconductivity
Ruslan Prozorov
This is from my little cookbook.
California Style Black-eyed Peas
Being a diverse, multi-ethnic, multi-racial family, we've added many traditions to our holiday celebrations. Black-eyed peas are a traditional African-American dish, served on New Year’s Day. We also serve black-eyed peas, along with tamales, on Christmas Day, right next to the turkey, mashed potatoes and cranberry sauce. My recipe is a bit different than the traditional recipe. I haven't had any complaints about it though!
Ingredients
1 bag of black-eyed peas
6 to 8 strips of pepper bacon
1 or 2 onions, chopped
1 can chunk tomatoes
1 small can chopped mild chiles
1/2 cup brown sugar
1 tsp crushed red peppers
2 tsp cumin
2 tsp garlic powder
Black pepper and salt to taste
Step 1:
Cook the black-eyed peas according to package directions. Drain.
Step 2:
While the black-eyed peas are cooking, chop the bacon into tiny pieces, add the chopped onions and fry until the bacon is almost crispy. Drain off the fat.
Step 3:
Add the bacon to the black-eyed peas, along with all the other ingredients. Do not drain off any liquids; pour all the juices into the pot. Bring to a boil, then turn the heat down and simmer for at least an hour, stirring occasionally.
Step 4:
Taste the black-eyed peas and add more sugar and/or spices if you like. Simmer another 15-20 minutes if you add more spices. Continue to simmer until the peas cook down and thicken, or serve right away.
Step 5:
Serve hot with rice, tamales or other family favorites.
Tips & Warnings
Use 4 to 6 cans of black-eyed peas if you prefer. Drain and then add all the other ingredients, following the directions from Step 2 on.
Use other leftover meats. Ham, turkey, chicken, even hamburger are all good.
If it's not hot enough for your family, use chopped jalapeños or more red peppers.
Careful with the crushed red peppers, they're hot.
 |
| Image Source: Link below |
Topics: Neutrinos, Particle Physics, Standard Model, Theoretical Physics
IN BRIEF
- The combined results of several studies have narrowed the potential range where a theoretical subset of particles known as sterile neutrinos could exist.
- The discovery of these fundamental particles could dramatically alter the Standard Model of particle physics.
THE HUNTED
Neutrinos are one of the most fascinating fundamental particles, mainly because they are so elusive. These barely detectable particles are almost massless and don’t interact with most anything. Studying neutrinos is not easy, and even less easy is studying a theoretical subset of the particles: sterile neutrinos.
Sterile neutrinos were initially proposed as a way to explain the Los Alamos National Laboratory experiments in the 1990s in which scientists discovered neutrino oscillations, the ability of neutrinos to change from one flavor to another. Based on what they expected from these potential particles, scientists determined a range of possible physical properties for sterile neutrinos, including their expected mass, but hadn’t yet actually confirmed their existence.
Futurism: The Dark Sector of Physics: The Hunt for New Fundamental Particles Is On
AUTHOR Dom Galeon, EDITOR Kristin Houser
Ok this is a short story i started a minute ago. Real gun shy about putting my written work out, but we're all friends here...lol
I am debating if i want to continue this and make it into a story then comic series.
comments are welcomed!
I followed behind my mentor closely as they made our way through the dark empty room. Boxes stacked up in no particular order littered the large warehouse. From what I could gather nothing else was housed here. If it were not for the few broken windows letting in the moonlight into the warehouse, they we were running into danger totally blind. And that could be dangerous.
It wasn't a particularly cold evening but as usual , the closer we got to our target the cooler the air seemed to get.
“Ok you can tell were are close now” My leader began to see the cold smoke come from his mouth as he spoke.
A shiver ran through my body when I noticed the symbol on the back of his neck suddenly began to glow.
“Heads up rookie” he looked back “You are about to get your first taste of a “Catcher”
I swallowed hard and wasn't sure if i was ready for this. But at this point it was not a choice.
“You feel it yet girl?” He asked.
“No” I said in a soft voice as my eyes strained through the low light. Then suddenly, a flash of green caught my eye and I could feel the sudden cold . My partner “Vet” says I was should be able to feel the nightmares essence as it struggled to break free. It knew why were here. It knew what we were all about.
But I was green, a rookie. I hadn't had the experience my mentor or the others had.
Walking slowly further in,a flash of green caught my eyes. I paused before calling out to Vet, as the creature glared at me with a bit of curiosity in it’s eyes. And there it was. It was bigger than I anticipated and that made me even more nervous. It was black….no, dark gray body it’s head misty in color with multiple eyes of deep green that illuminated in the darkness.
“Nice job rookie. Those fresh eyes are helpful”
They called him “Vet” because of his experience as a Catcher.
I have have heard that when a Nightmares gets caught it must be destroyed because of their volatile essence. They say it’s never pretty seeing a nightmare caught in a trap. It' snarls, snaps and spits, thrashes around and if you get too close it can that essence has been known to posses...or so I heard.
A few steps behind “Vet” I noticed his embedded artifact began to glow even brighter. “Vet” was ready to “catch” and “dispose”
Catcher's Artifacts can't be used by just anyone. You have to be attuned with it. It has to accept you as a user. Or be born with the ability.
Once it does they say, the ease at which you can sense and find Nightmares is a little unnerving
As the newbie I stared at it hopelessly and intrigued as the liquid like green lava spewed from it mouth while it snarled and snapped the closer “Vet” got to it.
It could have snatched Vet as close as he was to it. The Nightmare only reared it’s head up at him and growled.
It was fighting to untangle itself from the “trap” a triangle symbol that looked like it had webs crossing it in every direction. The trap was a symbol of a people lost long ago from what I understand. A symbol of what some believed was just a old tale to get kids to go to bed with happy thoughts. Whatever the reason, the symbol worked in catching these nightmares. Once the Nightmare has been dreamed it looks for an escape.
“Ok” Vet yelled unlocking and loading his gun without even missing a step, then quickly unloaded his clip on the “Nightmare”
The nightmare fought it's impending death, it's body starting slowly solidifying as it was caught in the trap. Heat from the enhanced weapon put him out of his suffering quickly. For a second you can see the nightmare in a solid form before it explodes into “dust” that settle onto the floor in a pile of shimmering green. Funny how beautiful the green “dust” that it leaves behind is. It shimmers without any light hitting it, it's actually a little sad.
“Vet” Stood still for a moment then took out a cigar and lit it taking along drag. Couldn't tell if he gave me a smile or a frown.
“Collect and let's get back to base Rookie” he exhaled a bloom of smoke.
Clean up isn't very hard. If you miss some it's not an issue. The dust just evaporates into the morning light. That's how they used to do it. Just catch and destroy and let the new day take care of the remains. But now retrieval is important.
You just scoop it all up with the special gloves and funnel that can withstand the extreme cold the dust gives off. I bottle it and bag it. Done!
Catchers don't know what exactly they do with the remains and it's not really none of their business.
“Good job! You hit on all sixes!” (slang for you nailed it) Vet stood over me as i unloaded my equipment
“So Rookie what ya think?”
“Not too bad it happens much quicker than I thought”
It's a job you try not to think too hard about other than doing your job and not getting hurt. I took the position because the pay was decent and it came with perks.
Living day to day trying to get by was become a chore. Most people tried to provide as best they could. Sometimes you closed your eyes to what was going on around you. either work one of the menial government provided job, found employment as best you could or scavenge in the streets .
Our elders were very few. Those who remember how this world fell apart or could remember the stories on how it happened were fading fast.
The rest of us were in the middle to try and reconstruct what once was.
I was a new Catcher was in my late twenties, single and if was lucky, be able to marry and reproduce. It seemed that I was person with qualifications suited for a “Catcher” profile perfectly. Healthy, well more healthy than most, had my wits about me and best of all didn't suffer from the sleep deprivation. I’m considered a lucky one...or bless if you wanted to look at it that way.
Like my new comrades, i didn't use the sleep medication that most everyone was dependent on now.
After re-adjust the strap on my bag the cold air left the room and was replaced the warm night air. It felt much better. My body didn’t particularly like the cold.
Exiting the warehouse to the vehicle the radio was flipped back on. Catchers run silent.communication cut off until you finish or need help. The radio seems to make your senses a little dull. You senses need to be on at all time.
The sudden shouting in their ears startled them both.
“Team 3 to base we need a back up”
Vet looked concerned with his cigar burning bright.
“Multiple Nightmare at our location, we need backup …..Old building on 6th”
Tossing his cigar out the door they rode off to Team 3's location.
“Guess your shift isn't over yet Rookie. You may get more that just “dust' on them virgin hands tonight”
This time of night the streets are empty. Not much to do in town well at least out in the open. Shops and markets are bolted shut. The makeshift street lights flicker on and off trying their hardest to light the way with limited power. People tend to stay inside and try to get as much sleep as possible.
Sleep.
A thing of value now.
Nights pass quickly and days are often too long, but what can you do. The earth has it way of doing things. And like a woman, after you treat her badly she's never the same.
Speeding through blocks, Vet opened his weapon bag.
“You got a weapon Rookie? Don't want you in a gunfight with bare knuckles”
“No one fights Nightmares with their bare hands Vet, but I'm strapped” I replied holding on tight through a hard right turn then coming to a stop
.
It was old building once stunning by the look at the outside decoration. The windows covered in with fine metal lines in straight lines that looked like a sunburst. Over the doorway were straight and symmetrical metal that met at the center top that formed a diamond shape with the most colorful glass inlay i’d ever seen.
Structurally it looked sound but it was old and decaying.
Even before they could get inside Team 3 was struggling over the radio. Whatever was going on inside they couldn't handle it.
“Team 1 to Team 3, what's your 20?
There was static at first, then yelling.
“Second floor …... you can't miss us!”
The static returned as both of them rushed inside and then up the stairs. There was swearing and gunfire. Vet's symbol was burning red and bright. Perhaps it was the adrenaline that got them us to the top of the stairs the so fast and into the fight. Vet unloading rounds before the I could even get from behind him.
5, no... 6...no 8 Nightmares snarling and snapping at us. Fangs bared and ready to strike. The Symbol failed unable to hold so many at once. The webbing that made it work , torn and dangling. There were piles of dust shimmering on the floor around it already destroyed by Team 3. How many had they destroyed? How many had come here?
I nervously yelled out “Is this supposed to happen?” retrieving holstered pistols. The Nightmares were acting like animals. Caged animals who were trying to get out. You could hear their hoarse voices,like a cat crying in the distance. It was eerie.
The room was freezing, between the loss of liquid nitrogen and one too many “Nightmares” ice crystals began to form on the walls. The temperature dropped as they kept firing.
“Why is it taking so long for these to go down?” A Team 3 member yelled out out while reloading.
“I got 3, but damn if they didn't use up most of my ammo”
It seemed like the harder they fought the harder they did, exhausting out ammo and running out of steam.
Vet looked over at me, his brow bunched up with concern. I could tell he was thinking of a way to end this quickly and get us out of here unharmed.
“Everyone down the stairs” we all quickly followed his lead and backed out slowly still engaging in gunfire.
“Rookie” he yelled out “How good a shot are you? Back me up!”
There was no time to answer. I quickly started to unload my clip while Team 3 made it quickly out of sight.
Then Vet reached into his pocket and brought out a silver orb. It beeped then suddenly was covered ice and smoke.
“Trust me Rookie?”
Yes, i trusted him. Did i really have a choice?
With a flick of his wrist he sent the orb flying towards the Nightmares that had grouped in the center of the room.
I saw the flash from the discharge, then the next second Vet's Face as he tackled me and we went tumbling down the stairs. The fall was slow..like time was holding us for a second. There was a loud boom then a swooshing sound, but it was overshadowed by the sound of us falling and me screaming as we hit the bottom floor and the room above us lit up in a bright flash.
Then the sudden fade to soft green, the luminous green of a defeated Nightmare.
Slightly stunned Vet rolled off m laid there beside my side for a second. Team 3, also exhausted sat quietly assessing the situation.
“You alive Rookie?”
“Yeah..I'll be fine” i finally got to my feet stretching out tight limbs. Vet re-lit another cigar and took a deep drag “ What the hell was that?”
None of us could answer. We were just as surprised as Vet. Two Nightmares in a trap, sure, three..ok it's been reported. But this many and this volatile, it left us all shaking our heads in wonder.
“This report is going to be a cluster fuck” Vet added finally on his feet and gathering his dispensed weapons. Base was going to want to know every detail and they were going to want every ounce of “dust” from this assignment.
Back at base, my night as a rookie Catcher was all anyone could talk about. All involved were given the day off to recover and be checked over by the doctors. Other Catchers began whispering about what happened. As i readied to leave i could feel all eyes on me and it made me uncomfortable.
Vet was already outside the gate to the base with a few other teammates, surrounded by females pawing all over him. His glowing cigar high in the air as he seemed revel in them fighting over his attentions.
Out of uniform I hadn’t noticed how big he was. The gear alone adds pounds to you and carrying it is like running a marathon.
For some reason I found myself staring a bit too long his way.
He caught my glance and smirked at me.
“Don’t get caught up in that hot mess Rookie” A female Catcher nudged me as we headed out together. “He’s got thirst that can’t be quenched” she chuckled “So sad!”
“Yeah” another Catcher responded “ That man’s got issues”
Passing Vet and his little group, I gave him a nod which he returned. I was glad this night was over. All i wanted was a good cup of tea and my bed.
Soon i was past the guard check mark and heading home. It was still dark out and the town was still sleeping.
The thud thud thud of my tires over the stone road that had be dug up to make way for a better one kept me alert. They were trying to get everything back together. Or at least as together as we could.
Home..finally.
Inside my tiny flat, I tired I not to think too much about tonight's events while making a cup of tea and settled into bed trying to shake off the pain of her fall.
At first, i thought I wouldn’t be able to sleep once home, but my body was telling me otherwise. Admittedly the sight of the Nightmare was still spinning in my head but sleep had more control this night.
Hearing about them and seeing them up close are two different things. They were fierce creatures no doubt but what had they really done to us?
I didn’t question if i could do this, because clearly i could. My feelings towards the nightmares, however, were neutral. But can i be neutral about something i’m supposed to destroy?
Chapter 1 end
 |
| Image Source: Technology Review |
Topics: Computer Science, Quantum Computer, Quantum Mechanics
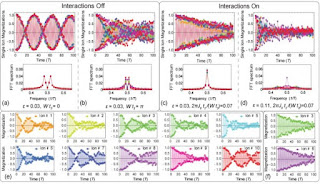
TECHNOLOGY REVIEW: Time crystals were first predicted in 2012. Now researchers have created time crystals for the first time and say they could one day be used as quantum memories.
Crystals are extraordinary objects, not least because of their symmetry. Crystals form repeating patterns that are the same in some directions but not all directions. That’s something of a surprise given that the laws of physics, which govern their formation, are the same in all directions.
That the laws of physics are spatially symmetrical but crystals are not is a phenomenon known as symmetry breaking. It comes about not by adding energy to a system, but by taking it away. Indeed, crystals are a manifestation of systems in their lowest energy states.
But the laws of physics are not only symmetrical in space but also in time. And that raises the interesting question of whether it is possible to break temporal symmetry in the same way. In other words, is it possible to create time crystals?
Abstract
Spontaneous symmetry breaking is a fundamental concept in many areas of physics, ranging from cosmology and particle physics to condensed matter. A prime example is the breaking of spatial translation symmetry, which underlies the formation of crystals and the phase transition from liquid to solid. Analogous to crystals in space, the breaking of translation symmetry in time and the emergence of a "time crystal" was recently proposed, but later shown to be forbidden in thermal equilibrium. However, non-equilibrium Floquet systems subject to a periodic drive can exhibit persistent time-correlations at an emergent sub-harmonic frequency. This new phase of matter has been dubbed a "discrete time crystal" (DTC). Here, we present the first experimental observation of a discrete time crystal, in an interacting spin chain of trapped atomic ions. We apply a periodic Hamiltonian to the system under many-body localization (MBL) conditions, and observe a sub-harmonic temporal response that is robust to external perturbations. Such a time crystal opens the door for studying systems with long-range spatial-temporal correlations and novel phases of matter that emerge under intrinsically non-equilibrium conditions.
Physics arXiv: Observation of a Discrete Time Crystal
J. Zhang, P. W. Hess, A. Kyprianidis, P. Becker, A. Lee, J. Smith, G. Pagano, I.-D. Potirniche, A. C. Potter, A. Vishwanath, N. Y. Yao, C. Monroe
 |
| Image Source: an ironically titled 2011 article on Salon.com: "Why Bob Dylan won't win the Nobel Prize" by Kevin Canfield |
Topics: Literature, Nobel Laureate, Nobel Prize
Press Release
13 October 2016
The Nobel Prize in Literature 2016
Bob Dylan
The Nobel Prize in Literature for 2016 is awarded to Bob Dylan
“for having created new poetic expressions within the great American song tradition”.
Bob Dylan was born on May 24, 1941 in Duluth, Minnesota. He grew up in a Jewish middle class family in the city of Hibbing. As a teenager he played in various bands and with time his interest in music deepened, with a particular passion for American folk music and blues. One of his idols was the folk singer Woody Guthrie. He was also influenced by the early authors of the Beat Generation, as well as by modernist poets.
Dylan moved to New York in 1961 and began to perform in clubs and cafés in Greenwich Village. He met the record producer John Hammond with whom he signed a contract for his debut album, called Bob Dylan (1962). In the following years he recorded a number of albums which have had a tremendous impact on popular music: Bringing It All Back Home and Highway 61 Revisited in 1965, Blonde On Blonde in 1966 and Blood On The Tracks in 1975. His productivity continued in the following decades, resulting in masterpieces like Oh Mercy (1989), Time Out Of Mind (1997) and Modern Times (2006).
Dylan’s tours in 1965 and 1966 attracted a lot of attention. For a period he was accompanied by film maker D. A. Pennebaker, who documented life around the stage in what would come to be the movie Don't Look Back (1967). Dylan has recorded a large number of albums revolving around topics like the social conditions of man, religion, politics and love. The lyrics have continuously been published in new editions, under the title Lyrics. As an artist, he is strikingly versatile; he has been active as painter, actor and scriptwriter.
"The Nobel Prize in Literature 2016 - Press Release". Nobelprize.org. Nobel Media AB 2014. Web. 13 Oct 2016
< http://www.nobelprize.org/nobel_prizes/literature/laureates/2016/press.html >
Topics: Materials Science, Nanotechnology, Spintronics
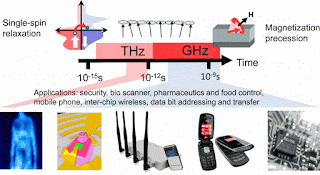
Abstract
This year the discovery of femtosecond demagnetization by laser pulses is 20 years old. For the first time, this milestone work by Bigot and coworkers gave insight directly into the time scales of microscopic interactions that connect the spin and electron system. While intense discussions in the field were fueled by the complexity of the processes in the past, it now became evident that it is a puzzle of many different parts. Rather than providing an overview that has been presented in previous reviews on ultrafast processes in ferromagnets, this perspective will show that with our current depth of knowledge the first applications are developed: THz spintronics and all-optical spin manipulation are becoming more and more feasible. The aim of this perspective is to point out where we can connect the different puzzle pieces of understanding gathered over 20 years to develop novel applications. Based on many observations in a large number of experiments. Differences in the theoretical models arise from the localized and delocalized nature of ferromagnetism. Transport effects are intrinsically non-local in spintronic devices and at interfaces. We review the need for multiscale modeling to address the processes starting from electronic excitation of the spin system on the picometer length scale and sub-femtosecond time scale, to spin wave generation, and towards the modeling of ultrafast phase transitions that altogether determine the response time of the ferromagnetic system. Today, our current understanding gives rise to the first usage of ultrafast spin physics for ultrafast magnetism control: THz spintronic devices. This makes the field of ultrafast spin-dynamics an emerging topic open for many researchers right now.
Journal of Applied Physics: Perspective: Ultrafast magnetism and THz spintronics
This Invited Perspective is part of the Special Topic “Cutting Edge Physics in Functional Materials” published in J. Appl. Phys. 120, 14 (2016).
Jakob Walowski1 and Markus Münzenberg1
Topics: Biology, Cancer, Nanotechnology, Research
My father died from complications due to smoking and lung cancer; my mother was a breast cancer survivor and two members of my wife's family are undergoing treatment for bone and breast cancer. In addition, a friend from college and my Calculus/Karate instructor in college both survived Melanoma. You can see how my attention and concern gravitates toward research like this.
Silica nanoparticles less than 10 nm in diameter in size could be used to kill cancer cells in a process known as ferroptosis according to new work by researchers at the Memorial Sloan Kettering Cancer Center in New York. The tumour-killing properties of the particles appear to be intrinsic to the particles themselves and as such they could be used as new therapeutic agents in their own right – without the addition of any cytotoxic molecules as is usually the case.
“The particles also appear to be well tolerated in normal biological tissue, so their cancer-cell-specific killing activity may represent a new therapeutic technique,” co-team leaders Michelle Bradbury and Michael Overholtzer tell nanotechweb.org.
The researchers performed their experiments on mice harbouring experimental tumours, including melanoma. They found that the silica nanoparticles, which were coated with ethylene glycol and functionalised with melanoma-targeting peptides, can bind to biological cells and deliver iron from their external environment into them. They thereby kill cancer cells by introducing large amounts of iron. This process is known as ferroptosis.
“The particles kill most efficiently when the cells have been starved, that is deprived of amino acids, which are essential cell nutrients,” explains Overholtzer. “For many cancers, their growth can outpace the development of a suitable vasculature, which is needed to deliver nutrients to the tumour. Indeed, tumour-associated vasculature is often dysfunctional or ‘leaky’ and as a result, cancer cells are known to experience periods where they do not receive enough nutrients to grow.
Nanotechweb.org: Silica nanoparticles suppress tumour growth, Belle Dumé
 |
| Image Source: Nobel Prize dot org |
Topics: Nobel Laureate, Nobel Prize, Peace
October 3: Physiology or Medicine
October 4: Physics
October 5: Chemistry
October 7: Peace
October 10: Economic Sciences
The Nobel Peace Prize for 2016
The Norwegian Nobel Committee has decided to award the Nobel Peace Prize for 2016 to Colombian President Juan Manuel Santos for his resolute efforts to bring the country's more than 50-year-long civil war to an end, a war that has cost the lives of at least 220 000 Colombians and displaced close to six million people. The award should also be seen as a tribute to the Colombian people who, despite great hardships and abuses, have not given up hope of a just peace, and to all the parties who have contributed to the peace process. This tribute is paid, not least, to the representatives of the countless victims of the civil war.
President Santos initiated the negotiations that culminated in the peace accord between the Colombian government and the FARC guerrillas, and he has consistently sought to move the peace process forward. Well knowing that the accord was controversial, he was instrumental in ensuring that Colombian voters were able to voice their opinion concerning the peace accord in a referendum. The outcome of the vote was not what President Santos wanted: a narrow majority of the over 13 million Colombians who cast their ballots said no to the accord. This result has created great uncertainty as to the future of Colombia. There is a real danger that the peace process will come to a halt and that civil war will flare up again. This makes it even more important that the parties, headed by President Santos and FARC guerrilla leader Rodrigo Londoño, continue to respect the ceasefire.
The fact that a majority of the voters said no to the peace accord does not necessarily mean that the peace process is dead. The referendum was not a vote for or against peace. What the "No" side rejected was not the desire for peace, but a specific peace agreement. The Norwegian Nobel Committee emphasizes the importance of the fact that President Santos is now inviting all parties to participate in a broad-based national dialogue aimed at advancing the peace process. Even those who opposed the peace accord have welcomed such a dialogue. The Nobel Committee hopes that all parties will take their share of responsibility and participate constructively in the upcoming peace talks.
"The Nobel Peace Prize 2016 - Press Release". Nobelprize.org. Nobel Media AB 2014. Web. 7 Oct 2016
< http://www.nobelprize.org/nobel_prizes/peace/laureates/2016/press.html >
Topics: Nobel Laureate, Nobel Prize, Physiology
I doubled up today due to yesterday's Netflix post. I'll follow the schedule as rolled out on NobelPrize.org:
October 3: Physiology or Medicine
October 4: Physics
October 5: Chemistry
October 7: Peace
October 10: Economic Sciences
Press Release
2016-10-03
The Nobel Assembly at Karolinska Institutet has today decided to award
the 2016 Nobel Prize in Physiology or Medicine
to
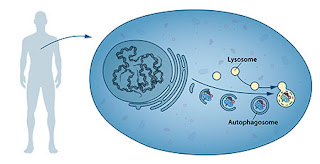
Yoshinori Ohsumi
for his discoveries of mechanisms for autophagy
Summary
This year's Nobel Laureate discovered and elucidated mechanisms underlying autophagy, a fundamental process for degrading and recycling cellular components.
The word autophagy originates from the Greek words auto-, meaning "self", and phagein, meaning "to eat". Thus,autophagy denotes "self eating". This concept emerged during the 1960's, when researchers first observed that the cell could destroy its own contents by enclosing it in membranes, forming sack-like vesicles that were transported to a recycling compartment, called the lysosome, for degradation. Difficulties in studying the phenomenon meant that little was known until, in a series of brilliant experiments in the early 1990's, Yoshinori Ohsumi used baker's yeast to identify genes essential for autophagy. He then went on to elucidate the underlying mechanisms for autophagy in yeast and showed that similar sophisticated machinery is used in our cells.
"The 2016 Nobel Prize in Physiology or Medicine - Press Release". Nobelprize.org. Nobel Media AB 2014. Web. 3 Oct 2016.
 |
| Image Source: DNA India |
Topics: Chemistry, Nobel Laureate, Nobel Prize
October 3: Physiology or Medicine
October 4: Physics
October 5: Chemistry
October 7: Peace
October 10: Economic Sciences
Press Release: The Nobel Prize in Chemistry 2016
5 October 2016
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry 2016 to
Jean-Pierre Sauvage
University of Strasbourg, France
Sir J. Fraser Stoddart
Northwestern University, Evanston, IL, USA
and
Bernard L. Feringa
University of Groningen, the Netherlands
"for the design and synthesis of molecular machines"
They developed the world's smallest machines
A tiny lift, artificial muscles and miniscule motors. The Nobel Prize in Chemistry 2016 is awarded to Jean-Pierre Sauvage, Sir J. Fraser Stoddart and Bernard L. Feringa for their design and production of molecular machines. They have developed molecules with controllable movements, which can perform a task when energy is added.
The development of computing demonstrates how the miniaturisation of technology can lead to a revolution. The 2016 Nobel Laureates in Chemistry have miniaturised machines and taken chemistry to a new dimension.
The first step towards a molecular machine was taken by Jean-Pierre Sauvage in 1983, when he succeeded in linking two ring-shaped molecules together to form a chain, called a catenane. Normally, molecules are joined by strong covalent bonds in which the atoms share electrons, but in the chain they were instead linked by a freer mechanical bond. For a machine to be able to perform a task it must consist of parts that can move relative to each other. The two interlocked rings fulfilled exactly this requirement.
The second step was taken by Fraser Stoddart in 1991, when he developed a rotaxane. He threaded a molecular ring onto a thin molecular axle and demonstrated that the ring was able to move along the axle. Among his developments based on rotaxanes are a molecular lift, a molecular muscle and a molecule-based computer chip.
Bernard Feringa was the first person to develop a molecular motor; in 1999 he got a molecular rotor blade to spin continually in the same direction. Using molecular motors, he has rotated a glass cylinder that is 10,000 times bigger than the motor and also designed a nanocar.
"The 2016 Nobel Prize in Chemistry - Press Release". Nobelprize.org. Nobel Media AB 2014. Web. 5 Oct 2016
< http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2016/press.html >
 |
| Image Source: The Guardian |
Topics: Nobel Laureate, Nobel Prize, Physics
October 3: Physiology or Medicine
October 4: Physics
October 5: Chemistry
October 7: Peace
October 10: Economic Sciences
Press Release: The Nobel Prize in Physics 2016
4 October 2016
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Physics 2016 with one half to
David J. Thouless
University of Washington, Seattle, WA, USA
and the other half to
F. Duncan M. Haldane
Princeton University, NJ, USA
and
J. Michael Kosterlitz
Brown University, Providence, RI, USA
”for theoretical discoveries of topological phase transitions and topological phases of matter”
They revealed the secrets of exotic matter
This year’s Laureates opened the door on an unknown world where matter can assume strange states. They have used advanced mathematical methods to study unusual phases, or states, of matter, such as superconductors, superfluids or thin magnetic films. Thanks to their pioneering work, the hunt is now on for new and exotic phases of matter. Many people are hopeful of future applications in both materials science and electronics.
The three Laureates’ use of topological concepts in physics was decisive for their discoveries. Topology is a branch of mathematics that describes properties that only change step-wise. Using topology as a tool, they were able to astound the experts. In the early 1970s, Michael Kosterlitz and David Thouless overturned the then current theory that superconductivity or suprafluidity could not occur in thin layers. They demonstrated that superconductivity could occur at low temperatures and also explained the mechanism, phase transition, that makes superconductivity disappear at higher temperatures.
"The 2016 Nobel Prize in Physics - Press Release". Nobelprize.org. Nobel Media AB 2014. Web. 4 Oct 2016.
< http://www.nobelprize.org/nobel_prizes/physics/laureates/2016/press.html >
 |
| Image Source: VIBE |
Topics: African Americans, Diaspora, Diversity, Diversity in Science, Science Fiction
Marvel Comics has the distinction of diversifying American Mythology (comics) first with The Black Panther, Luke Cage et al, and it would be a while before DC Comics "got the memo." As is now evidenced in real-time after the Agents of Shield, Ant-Man, Avengers, Captain America, Guardians of the Galaxy, Hulk, Iron Man franchises and the soon-coming Black Panther and (yet another) Spider-man reboot, DC still appears to be behind the curve. "Black Lightning" and "Static Shock" seem to be clones of one another, as well as going from Hal Jordan to Jon Stewart as Green Lantern, ostensibly selected as a "backup." Mr. Terrific has had several incarnations in comics and on the CW's Arrow series. Cyborg, who will be in the Justice League movie, is an interesting yet mechanized character, more machine than man and gives rise to questions of his own humanity as well as Hollywood and technology's power over black bodies.
The latest is Luke Cage on Netflix, which had the distinction of being so popular this weekend it crashed their site [1]. An inquiry online found at least one friend had the same experience during our weekend's binge watching. I checked my credit card, and Netflix had taken its usual monthly fee on schedule.
There is as with any retelling some similarities and a lot of differences between the original character and the newer version. Such as: Luke had an Afro and "talked a lot of jive," which resonated with the audience Marvel was trying to reach at that time. It definitely did with me and my friends. This Luke reads a lot of black literature with a clear knowledge of self and history, and is a subtler, hipper version of the now defunct (but in DVD format) "Schoolhouse Rock."
Netflix alludes to his previous career in law enforcement that wasn't in the original version, which explained his tracking abilities pre his eventful "accident," aided by a rabidly racist guard. The "science" or fiction thereof as anything else in Marvel stems from the Super Soldier serum that created Captain America and most of the Marvel superhero pantheon. His prison break was as I remembered, but his homage to how "Luke Cage: Hero for Hire" used to look was brief and hilarious to witness! I won't spoil it, but if you're confused, Google some old classic images of him, and compare it to Mike Colter's enactment. As the actor eludes, Harlem is as much a character in the series [2], a subtle history of literature and heroes like Crispus Attucks, Malcolm X, David Dinkins and others name-dropped for a new generation of millennials that have lost faith in traditional institutions like government, church and Civil Rights leaders, who may be ready for an impervious Steve Biko from "around-the-way."
The shear brilliance of a bullet-proof black superhero in light of black bodies falling in the streets is ironically well-timed; as artful as starting a lot of the stories from their end, and filling in the blanks the rest of the episode, a respite from what the author Touré (last week) filling in on the Karen Hunter Show on Sirius XM aptly referred to their sheer repeated orgy and negative psychological impact as "snuff films." Netflix's Luke Cage series gives those of us affected in our spirits a world of larger-than-life heroes as well as the quintessential villains (Cottonmouth) as respite to momentarily escape to.
"We are one bullet away from being a hashtag" is an oft-repeated meme as frustration builds when media follows the typical formula after the pending hashtag expires from life: any infraction with the law, even jaywalking must be trotted out as an evidence and reason for the public execution by the state. From a brief humorous cameo in his former seventies hero garb; to the homage to Trayvon Martin slaughtered by an unqualified idiot (whose name I won't type or mention). The Luke Cage for THIS age like his original version has no mask, disguise or secret identity. He's a man as "Pops" inspired him to be, "going forward" garbed in nothing but grit, bullet-proof skin, super-powered muscles, righteous indignation, honor, nobility...
And a HOODIE! We are all Luke Cage. #HoodieUp
1. Entertainment: Marvel's Luke Cage is so popular, it actually caused Netflix to crash
2. NBC News: 'Luke Cage' Stars, But Harlem is Second Lead in Netflix Series
 |
| Image Source: Link below |
Topics: Climate Change, Existentialism, Global Warming
I can callously not care: after all, giving patterns in my own family, I'm doubting I'll be here in 2050 (88) or 2100 (138) especially. I would like to think my remains - reclaimed in promession - will be aiding a Maple tree with nourishment and growth.
However, there is a strong possibility my children will be here, and so will in turn any children they may procreate.
Resources are strained as we have overachieved in the Biblical mandate "be fruitful and multiply"in a society that encourages consumption, eschews conservation and praises waste. This naturally leads to income disparity and hoarding and callousness by the 1%. John Calhoun's experiments with our rodent cousins he wrote up in 1962 (joy: the year of my birth) was likely used as source material for many Dystopian novels. The bridge from Utopian to Dystopian society had the coined apropos name "behavioral sink."
Despite the best wishes of Stephen Hawking, we're not at the point of sub-light or warp travel. And, what about our behaviors would change to where we wouldn't become "Manifest Destiny" intergalactic locusts, in due time with colonial capitalism ravage yet another habitable planet?
I can't "complete the circle of life" nourishing a tree on a dysfunctional planet.
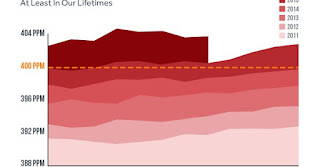
The bad news: Earth's climate change problem just passed a point of no return. Atmospheric carbon levels have passed 400 parts per million, and they won't return to more environment-friendly levels "ever again for the indefinite future."
The good news? Oh, wait, no. Sorry. We're pretty screwed.
We already knew it was bad. After all, in the last 20 years, humanity destroyed 1.27 million square miles—10 percent—of the Earth's wilderness. Climate change has "devastated" 93 percent of the Great Barrier Reef. The world's so screwed that genius Stephen Hawking recently claimed that "the human race has no future if it doesn't go to space."
But now we really, really know we're in trouble. Scientist Ralph Keeling, who's in charge of the Scripps Institute for Oceanography's carbon dioxide monitoring program, wrote in a blog that "it already seems safe to conclude that we won’t be seeing a monthly value below 400 ppm this year—or ever again for the indefinite future."
Similarly, NASA's chief climate scientist Gavin Schmidt told Climate Central, “In my opinion, we won’t ever see a month below 400 ppm.”
A lot of problems come with climate change. Because of it, one-fourth of the Earth's species could be extinct by 2050. It also screws up food webs, as polar bears are finding out the hard way. Millions and millions of people will have to relocate due to rising sea levels, with scientists estimating that over 13 million Americans might have to move by 2100.
Complex:
Enjoy Earth While It Lasts: Atmospheric Carbon Levels Pass the Point of No Return
Mac MacCann
Going to try for a inked piece of art every day for 31 days...wish me luck